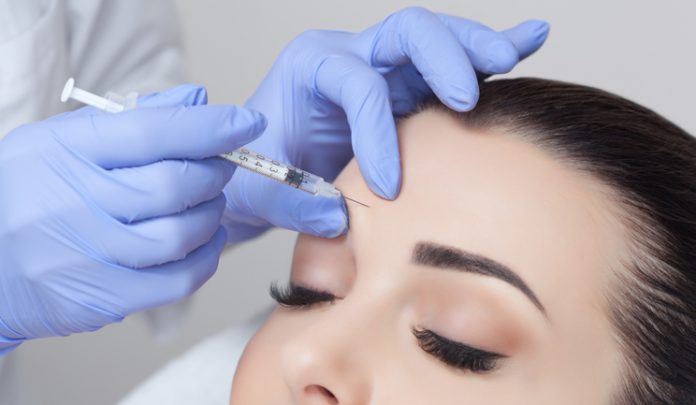
The results of two studies conducted by pharmaceutical company Galderma (UK) Limited have demonstrated high patient satisfaction for the use of Azzalure (AbobotulinumtoxinA), its botulinum toxin type A product, in the glabellar complex.
The first study, titled the ANGEL study (n=533), took place in 66 centres across four countries, and demonstrated that 97.4% of patients were satisfied or very satisfied with the aesthetic outcome with Azzalure (AbobotulinumtoxinA) at three weeks, and 89.6% were satisfied or very satisfied at four months.
The second study, the APPEAL study (n=135), took place in 13 centres in six countries and highlighted that 97% of patients treated were satisfied or very satisfied three weeks after their first Azzaure (AbobotulinumtoxinA) glabellar line treatment and this figure increased to 99.3% by the third treatment.
Patricia Ramos, brand manager, medical solutions, aesthetic and corrective products, prescription division, Galderma UK and Ireland, said, “It is very important for clinics to have a product that they can trust. These patient satisfaction studies add to the wealth of clinical data that we have demonstrating Azzalure’s (AbobotulinumtoxinA) efficacy.”
Full prescribing information for Azzalure® (AbobotulinumtoxinA) can be found here.