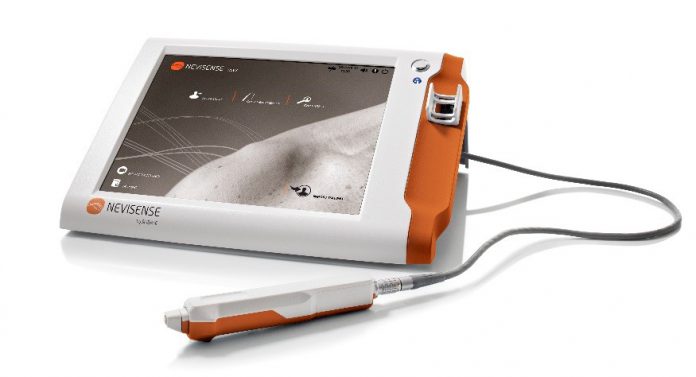
The FDA has approved SciBase AB’s Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection. Nevisense, an AI-based point-of-care system for the non-invasive evaluation of irregular moles remains the only FDA approved system available for melanoma detection in the US.
Nevisense 3.0 is a more efficient and streamlined version of the product previously approved by the FDA. The European version released at the end of 2018 has significantly improved both adoption and utilization, and has been used clinically on over 30,000 patients to date.
“The release of Nevisense 3.0 in late 2018 in Germany and the EU has been the catalyst for five consecutive quarters of growth, and we see this as step one of our strategic plan” says Simon Grant, Chief Executive Officer of SciBase. “Receiving US approval means that we can focus on step two of our strategy which is to increase our marketing and sales activities in the US based on our deep market experience with Nevisense 3.0 in Germany and the positioning we have developed for Nevisense in the US.”