Cynosure announce the launch of the Potenza™ radiofrequency (RF) microneedling device, the first and only FDA-cleared four-mode RF microneedling device offering clinicians unrivaled versatility and personalized treatments for patients.
Launching in Autumn 2020, the new standard in RF microneedling, the Potenza device’s four modes offer more customized microneedling treatments for patients than ever before and allow practitioners to deliver both shallow and deep treatments on a single system. The device’s monopolar RF mode delivers energy across a large area of tissue for deep heating and skin tightening through soft tissue coagulation, not only on the face, but anywhere on the body. The bipolar RF mode offers more concentrated delivery of energy to treat superficial tissue and provide ideal skin revitalization results. The groundbreaking device is also equipped with Tiger Tip™ technology, the first semi-insulated needles of its design which allow practitioners to expand the treatment zone and address more tissue per treatment, which translates to quicker sessions for patients, without sacrificing the epidermis. The device is effective on Acne Vulgaris and is also armed with a single-needle handpiece designed to target and improve blemishes.
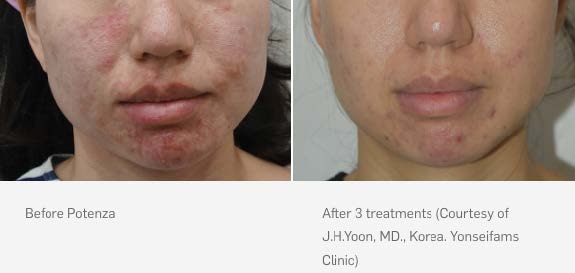
Potenza treatments use ultrafine needles and radiofrequency energy to penetrate the top layer of the skin and trigger the body’s natural healing process to regenerate new collagen and elastin. Unlike some other skin revitalization options on the market, Potenza treatments can be performed on all skin types, anywhere on the body and any time of year and even use on Acne Vulgaris.
“Our goal at Cynosure is to provide our customers with revolutionary technologies so they can consistently deliver outstanding results,” said Todd Tillemans, Chief Executive Officer of Cynosure. “Potenza takes the microneedling category to a new level by offering unprecedented flexibility for doctors, which translates to personalized treatments and satisfied patients with exceptional outcomes.”
About Cynosure
Cynosure develops, manufactures and markets aesthetic treatment systems that enable plastic surgeons, dermatologists and other medical practitioners to perform non-invasive and minimally invasive procedures for skin revitalization, hair removal, body contouring, women’s health, treat vascular and benign pigmented lesions, remove multi-colored tattoos, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus and ablate sweat glands. Cynosure’s product portfolio is composed of a broad range of energy sources including Alexandrite, diode, Nd: YAG, picosecond, pulse dye, Q-switched lasers, intense pulsed light and RF technology. Cynosure sells its products globally under the Cynosure, Palomar, ConBio and Ellman brand names through a direct sales force in the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea, and through international distributors in approximately 130 other countries.