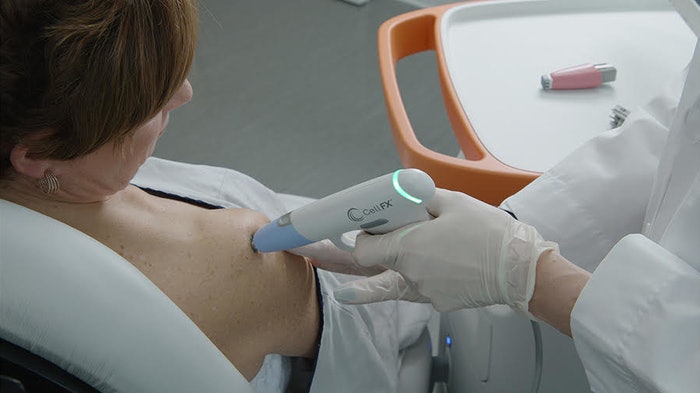
The FDA has cleared the CellFX® System from Pulse Biosciences, Inc. for dermatologic procedures requiring ablation and resurfacing of the skin, and a controlled commercial launch will soon beginning in the US with a of select group of leaders in aesthetic dermatology.

The CellFX System is a multi-application platform that harnesses Pulse’s proprietary NPS technology delivering nano-second pulses of electrical energy to non-thermally clear cells while sparing adjacent noncellular tissue. NPS technology clears unwanted cellular structures while limiting collateral damage to surrounding healthy skin, supporting aesthetically pleasing outcomes.
“The CellFX System offers a unique non-thermal mechanism that in my experience can clear epidermal and mid-dermal cellular structures without damaging the non-cellular dermal collagen, which can lead to remarkable improvements in common skin problems that I see every day,” says Brian Zelickson, MD, Founder and Medical Director, Zel Skin and Laser Specialists in Edina, MN. “We look forward to adding the CellFX System to our practice and foresee a promising future of new applications for this versatile technology platform.”
President and Chief Executive Officer of Pulse Biosciences Darrin Uecker calls FDA clearance for the CellFX System, “a tremendous achievement for Pulse Biosciences” and part of a stepwise approach with FDA. “This continued progress on our regulatory strategy, following the recent receipt of the CE mark, is another strong validation of the safety and efficacy of our technology. We are proud of our team and thankful to the investigators and FDA for their diligent efforts as part of this accomplishment,” he adds. “We look forward to proceeding with our Controlled Launch program with KOLs in the United States. This measured approach to launching the CellFX System is our top priority for 2021 and will be key to long-term commercial success of the CellFX platform.”