It’s October and we are celebrating Breast Cancer Awareness Month. Usually, when talking about breast cancer, most of the conversations gear towards treatments for the condition, such as mastectomy or breast removal surgery. Also, we hear about the amazing stories of breast cancer survivors, most of them undergoing the mentioned surgery.
However, what many do not mention is what happens afterwards. Some breast cancer survivors who have undergone mastectomy go through an emotional slump after their operation. As they have lost their breast, they tend to feel less like a woman. It’s such a common occurrence among these women that procedures such as breast reconstruction have become popular.
Breast reconstruction helps to rebuild the shape and look of the breast. With that said, breast reconstruction has a different process and medical needs compared to your ordinary breast augmentation procedure. There is little to no breast tissue to work with breast reconstruction as most of it has been removed due to mastectomy. So, it would be difficult to place implants- of any size or shape- into the chest area, and forcing them to do so may cause major damage to the tissues or the implant itself. Luckily, there are tissue expanders that can be used to aid this type of situation.
BreastRecon™ can help women feel like themselves again, by bringing them solutions that enhance both beauty and safety.
Meet the Motiva Flora® Tissue Expander!
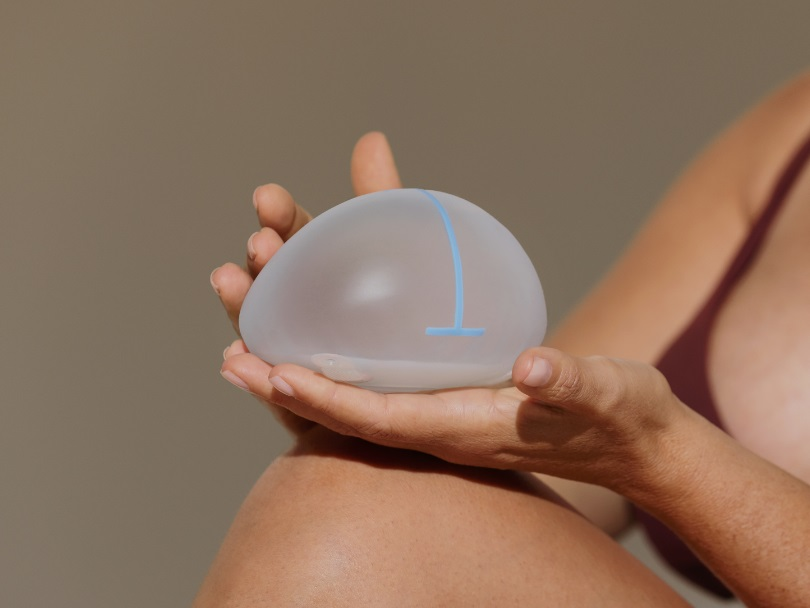
Motiva Flora® is a revolutionary MRI conditional tissue expander, meaning it is not contraindicated during MRI scanning2.
Constructed with state-of-the-art technologies, this tissue expander provides women, undergoing two-stage breast reconstruction, safety and stability during the tissue expansion process3,8.
State-of-the-art Technology

MRI conditional technology
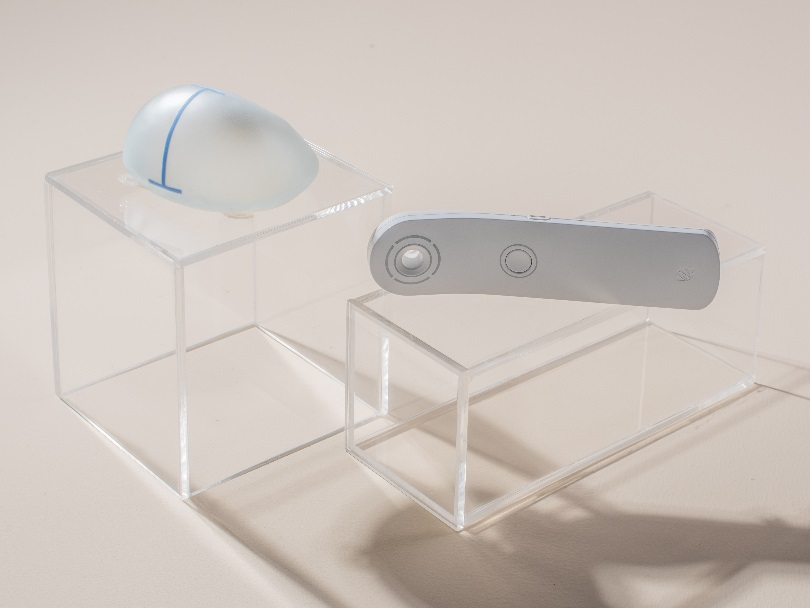
Conventional tissue expanders may have complications with their magnetic components during vital MRI scanning. As a result of these complications, conventional tissue expanders have been classified as unsafe for MRI scanning.
The Motiva Flora® Tissue Expander was purposely built with a nonmagnetic port in its detection system to offer women a safe solution to MRI scanning during the tissue expansion process.
The advanced technology of the RFID port holds MRI conditional labeling, thus proven to reduce drawbacks seen in conventional tissue expanders2,10.
What makes us unique:
- Breakthrough Technologies
- Nonmagnetic-port detection system
- MRI Scanning Compatible
Hybrid ergonomic reconstruction
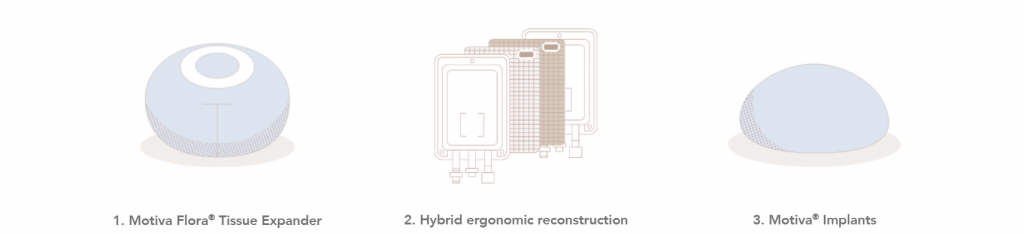

Implant-based reconstruction (IBR) is a more popular procedure for multiple reasons: reduced donor site mobility, reduced scarring, and shorter operating and recovery times in comparison to autologous reconstruction13.
Hybrid ergonomic reconstruction is the concept of combining autologous tissue with Motiva® Implants, offering patients the option of a natural-looking result14.

References
1 Breast Cancer: Types of Treatment | Cancer.Net. Accessed July 27, 2021. https://www.cancer.net/cancer-types/breast-cancer/types-treatment
2 Breast Tissue Expander With Radiofrequency Identification Port: Assessment of MRI Issues | Enhanced Reader.
3 D’Onofrio C. Subfascial Breast Augmentation with Crossed Fascial Sling, Under Tumescent Anaesthesia With or Without Sedation and Lower Periareolar Access. Aesthetic Plastic Surgery. Published online 2020. doi:10.1007/s00266-020-01723-0
4 Rigo MH, Piccinini PS, Sartori LDP, de Carvalho LAR, Uebel CO. SMS—Split Muscle Support: A Reproducible Approach for Breast Implant Stabilization. Aesthetic Plastic Surgery. Published online 2020. doi:10.1007/s00266-019-01565-5
5 Doloff JC, Veiseh O, Mezerville R de, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering 2021. Published online June 21, 2021:1-16. doi:10.1038/s41551-021-00739-4
6 Quirós MC, Bolaños MC, Fassero JJ. Six-year prospective outcomes of primary breast augmentation with nano surface implants. Aesthetic Surgery Journal. Published online 2019. doi:10.1093/asj/sjy196
7 Establishment Labs. Post Market Surveillance Report Q4 December. Published online December 2020.
8 Khavanin N, Gust MJ, Grant DW, Nguyen KT, Kim JY. Tabbed Tissue Expanders Improve Breast Symmetry Scores in Breast Reconstruction. Archives of Plastic Surgery. 2014;41(1):57. doi:10.5999/APS.2014.41.1.57
9 Clemens MW, Fairchild B, Ellsworth W, et al. Breast Surgery Safety and Efficacy of Smooth Surface Tissue Expander Breast Reconstruction Level of Evidence: 4. Aesthetic Surgery Journal. 2020;40(1):53-62. doi:10.1093/asj/sjy199
10 S Y, BN J, DJ W, ER P, NM H. Recognizing artifacts and optimizing breast MRI at 1.5 and 3 T. AJR American journal of roentgenology. 2013;200(6). doi:10.2214/AJR.12.10013
11 Breast Implant Reconstruction: Surgery details, implant types, risks and more. Accessed July 27, 2021. https://www.breastcancer.org/treatment/surgery/reconstruction/types/implants
12 Bertozzi N, Pesce M, Santi P, Raposio E. Tissue expansion for breast reconstruction: Methods and techniques. Annals of Medicine and Surgery. 2017;21:34. doi:10.1016/J.AMSU.2017.07.048
13 The American Cancer Society medical and editorial content team. Body Image and Sexuality After Breast Cancer. Published 2019. Accessed July 27, 2021. https://www.cancer.org/cancer/breast-cancer/living-as-a-breast-cancer-survivor/body-image-and-sexuality-after-breast-cancer.html
14 FBJL S, B L, K VL, PN B. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plastic and reconstructive surgery Global open. 2020;8(7). doi:10.1097/GOX.0000000000002966
15 EB K, LP B. Fat Grafting to the Breast: Clinical Applications and Outcomes for Reconstructive Surgery. Plastic and reconstructive surgery. 2017;140(5S Advances in Breast Reconstruction):69S-76S. doi:10.1097/PRS.0000000000003945
This information is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Motiva® does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it in a procedure or surgery. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Motiva® product. The information presented is intended to demonstrate particular products, as well as the breadth of Motiva® product offerings. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your local representative if you have questions about the availability of specific products in your area. Motiva® products that are CE marked are marked according to the applicable EU Regulations and Directives.